Sparkling Water Under Nanoconfinement
A research team led by EI faculty Prof. Ding Pan, Associate Professor of Physics and Chemistry, gave tips to unveil the mysteries of carbon-bearing fluids in deep Earth.
Dissolving CO2 in water is an everyday process, but its ubiquity belies its importance. It has great implications for Earth’s carbon cycle, which deeply affect global climate change and sustainable development of human society. In carbon capture and storage efforts, turning CO2 together with water into rocks offers a secure method to permanently store carbon underground with a low risk of return to the atmosphere.
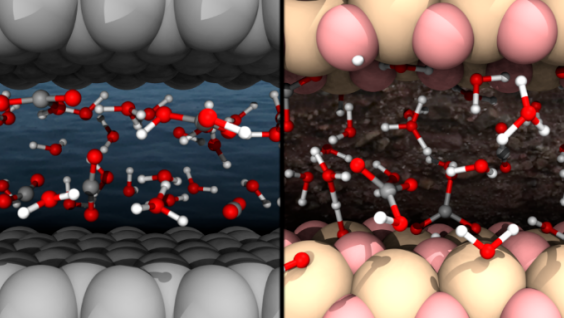
Previous studies more focus on properties of dissolved carbon in bulk solutions; however, in deep Earth or underground carbon storage, aqueous solutions are often confined to the nanoscale in pores, grain boundaries, and fractures of Earth’s materials, where spatial confinement and interface chemistry may make the solutions fundamentally different.
Prof. Ding Pan and his PhD students, Nore Stolte and Rui Hou, applied long-time ab initio molecular dynamics simulations to study the CO2 reactions in water. Their simulations are based on first principles in physics, and do not require any experimental or empirical input. The simulation results can be used to guide the following experiments. They compared the carbon solutions nanoconfined by graphene, an atomic layer of graphite, and stishovite, a high pressure SiO2 crystal, with the bulk solutions. The graphene layer is hydrophobic, while the stishovite surface is hydrophilic. They found that CO2 reacts more in nanoconfinement than in bulk. The stishovite-water interface makes the solutions more acidic, which may hinder the reactions of CO2.
Their research suggests that CO2 may be more active than previously thought in Earth’s deep carbon cycle, which greatly influence the carbon budget in Earth’s near-surface reservoirs. Confining CO2 and water in suitable nanoporous minerals may enhance the efficiency of underground carbon storage. This study provides a fundamental step toward unveiling the mysteries of carbon bearing fluids in Earth’s interior. The team’s work “Nanoconfinement facilitates reactions of carbon dioxide in supercritical water” was recently published in Nature Communications.
Prof. Ding Pan is jointly appointed in the HKUST Department of Physics and Department of Chemistry. His Angstrom group develops and applies high-performance first principles and machine learning methods to seek answers to the urgent and fundamental scientific questions relevant to sustainable development, e.g., water science, deep carbon cycle, and clean energy. http://angstrom.ust.hk/
Reference:
Stolte, N., Hou, R. & Pan, D. Nanoconfinement facilitates reactions of carbon dioxide in supercritical water. Nat Commun 13, 5932 (2022).